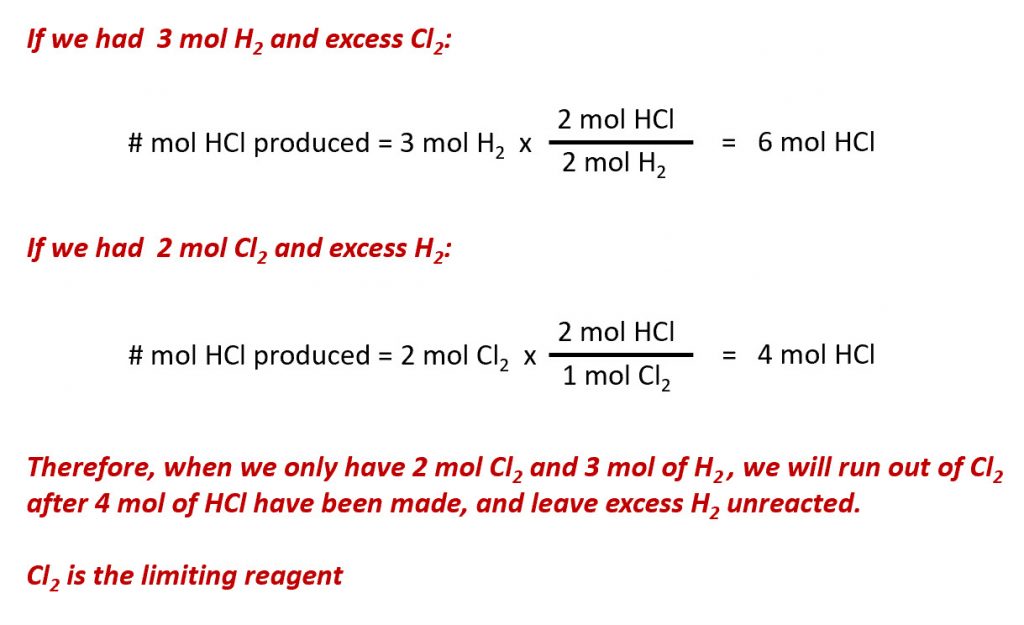
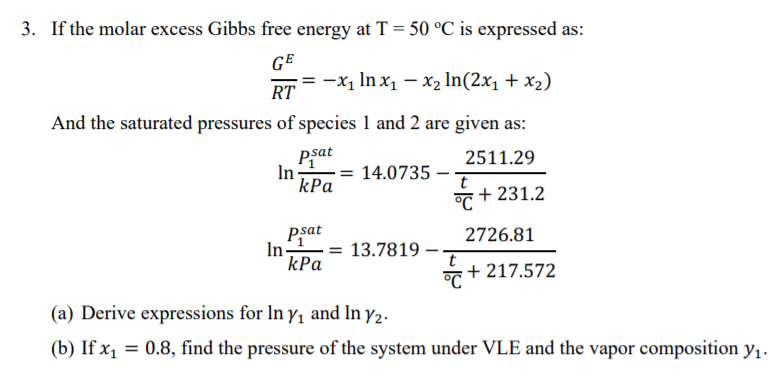
4. The excess molar Gibbs free energy of formation of solid solutions in the system Au-Cu can be represented by GX = -28280 XauXcu (J) Calculate the activities of Au and Cu
R ational Interpolation F orm ulae for the Excess Functions o f B inary M ix tu res H. Brodowsky Institut für Physikalische Che
Derived thermodynamic properties of binary mixtures of m-Xylene, o-Xylene, and p-Xylene, with N,N-Dimethylformamide at T = (293.15, 303.15, 313.15 and 323.15) K : Oriental Journal of Chemistry
6. 280 g of a mixture containing CA , and C_{2}H_{ 5:2 molar ratio is bumt in presence of excess ofoxygen. Calculate total moles of Co_{2} produced.(A)9 (B)T8 (C)7 (D) 12The | Snapsolve

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

OneClass: Acetylene gas (C2H2) is burned completely with 20 percent excess air during a steady state-...